

June 2020: Jared Soto Ruiz joins group as an LSAMP Scholar working on the phosphate sequestration project
May 2020: Dr. Clear awarded faculty research grant (FRG) from IU South Bend Academic Affairs for summer research
Jan 2020: Lab acquires new (to us) Biotage Isolera One automated chromatographic purification system...now we just need some columns...
July 2019: Dr. Clear starts a new position as Assistant Professor of Chemistry at Indiana University South Bend in the Department of Chemistry & Biochemistry
May 2019: Gena and Natalie graduate and move on to bigger and better things!
April 2019: Natalie Jarret presents her research during Scholars Week and defends her thesis
February 2019: Gena Wilson presents her research at SURC 2019
November 2019: Dr. Clear presents his research at the Univeristy of Notre Dame
May 2018: Shelby and Hannah graduate and move on to bigger and better things!
March 2018: Dr. Clear and students Hannah Adamson and Shelby Wakefield present research at the Spring 2018 ACS National Meeting in New Orleans, LA
September 2017: The Clear group is awarded startup funding from the KY NSF EPSCoR
May 2017: The Clear group receives an MSU CISR grant
February 2017: The Clear group receives the 2017 Marshall and Annette Gordon chemistry research grant
Work in the Clear lab integrates themes from organic, physical, and analytical chemistry to address questions regarding recognition of biologically important anions.is in the area of bioorganic and supramolecular chemistry, with general interests in the following areas:
Importantly, students participating in research will be exposed to a range of techniques, from traditional organic synthesis to binding studies requiring repetitive measurement and extensive data analysis. As a result, this research experience will help train students for graduate and professional studies as well as careers in industry.
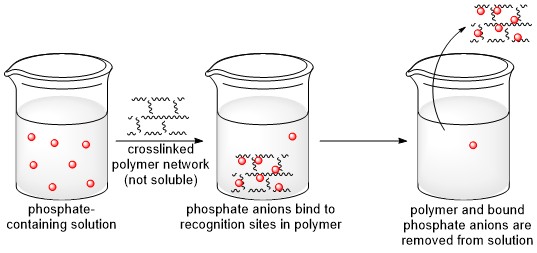
A. Polymeric Materials for Phosphate Removal from Water
Phosphate is an essential nutrient for health in all living organisms. However, elevated levels of phosphate and phosphate derivatives can have destructive effects on human health and on the environment. There is a need for agents that can remediate these issues by sequestering the phosphate species from water through adsorption to an insoluble nanostructure and removing the anions from the solution, as illustrated below. This need is evidenced in part by the large number of recent studies being published on the topic, and there is particular interest in developing effective and efficient strategies for removing phosphate from both environmental water and wastewater. We are currently synthesizing new polymeric networks and studying their capacity for binding phosphate and to learn about the structural determinants of binding capacity, phosphate selectivity, and degree of swelling. The reference point for the studies will be the polyammonium structure found in sevelamer hydrochloride, and the effect of replacing some or most of the ammonium (charged amino) groups in the polymer with guanidinium (charged guanidino) groups will be examined.
Techniques/Instrumentation: organic synthesis, materials characterization, UV/Vis absorbance spectroscopy, binding analysis
Additional Reading:
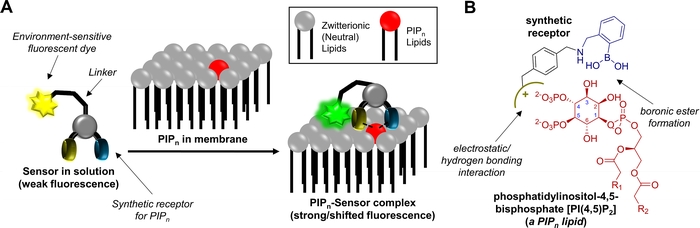
B. Sensor for Anionic Signaling Lipids
The main objective of this work is to generate low molecular weight sensors with the ability to cross cell membranes, bind to phosphatidylinositol phosphate lipids (PIPn) in the membrane, and report the binding event through a fluorescent response. The PIPn lipids are a family of biomolecules that play crucial roles in communication within and between living cells. PIPn lipids are usually found on the interior membranes in animal cells, and their localization within cells and dynamic changes in their distribution are the basis for many cell signaling events. It is also known that dysregulation of PIPn levels is a feature of many diseases. Sensors with the ability to recognize and bind PIPn have the potential to be powerful research tools for understanding key signaling pathways, protein-ligand interactions, and protein-lipid interactions. Thus far, most PIPn sensors have been fluorescently labeled protein or antibody receptors that image localization and dynamics of the lipids. A significant issue with protein-based PIPn sensors is that they have to be introduced by either genetic expression or microinjection for use in cellular studies since proteins cannot diffuse across the cell membrane and the lipids are typically present within cells and interior membranes. We are studying sensors that consist of a membrane permeable synthetic receptor (SR) designed to selectively recognize and bind to the inositol phosphate (IPn) headgroup on the lipid linked to a fluorescent dye that changes signals when it is brought to the membrane surface through the lipid-receptor interaction. Our underlying structural hypothesis of this proposal is that synthetic receptors with combinations of phosphate recognition units and boronic acids, will retain unique affinity for different PIPn while retaining the ability to cross cell membranes.
Techniques/Instrumentation: organic synthesis, chromatography, NMR spectroscopy, fluorescence spectroscopy, liposome preparation, binding analysis
Further reading:
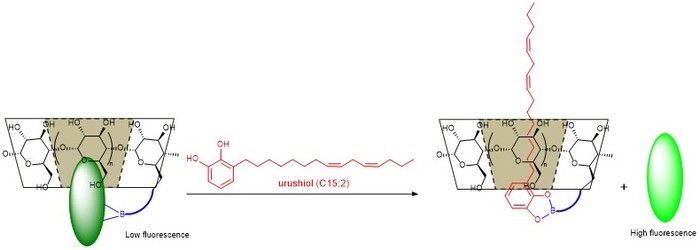
C. Recognition and Sensing of Urushiol
Allergic contact dermatitis (ACD) is caused by exposure to urushiol, an oily sap derived from poison ivy/oak/sumac, and it affects approximately 50 million people annually. Urushiol is composed of a catechol functional group bound to a hydrocarbon chain with varying length and degrees of unsaturation. The sensitivity to urushiol is cause by the absorption of this sap through the skin where it binds to proteins and elicits an allergic response leading to a rash and itching. Although prevention of exposure to urushiol is recommended, it is difficult to prevent exposure since urushiol can be transferred by contaminated surfaces such as animal fur, clothing or outdoor tools and those exposed may not be aware until their body has already begun to mount a allergic response. In the lab, we are synthesizing urushiol and studying its encapsulation by cyclodextrin, a cone-shaped oligosaccharide. We are also investigating the effect of boronic acid functionalization of the cyclodextrin to increase the affinity and selectivity for urushiol over other lipid-like molecules. Eventually, the group would like to create a sensor to detect urushiol on surfaces.
Techniques/Instrumentation: organic synthesis, chromatography, NMR spectroscopy, fluorescence spectroscopy, binding analysis
Further reading:

David Aupperlee (Chemistry, IU South Bend)
April Kinney (Biological Sciences, IU South Bend)
Jared Soto Ruiz (Chemistry, IU South Bend)
Danielle Voss (Biological Sciences, IU South Bend)
Hannah Wallace (Biological Sciences, IU South Bend)
Ryan Garcia (Chemistry, Murray State)
Josh Batson (Chemistry, Murray State)
Erin Calvert (Chemistry, Murray State)
Natalie Jarrett (BS Chemistry, Muray State)
Gena Wilson (BS Chemistry, Murray State); Current Position: Biochemistry Ph.D. program at Univeristy of Notre Dame
Bailey Morales (BS Chemistry, Murray State)
Hannah Adamson (BSA Vet Tech); Current position: DVM program at Ross University School of Veterinary Medicine
Shelby Wakefield (BS Chemistry); Current position: Chemistry Ph.D program at University of Wyoming
Dmitriy Bachynsky, graduate student
Jacob Meadows, B.S. Chemistry
Motivated undergraduate students who are interested in doing research are welcome to join. If you are interested, contact Dr. Clear by e-mail (kclear@iusb.edu) or just stop by his office (NS 041).
Graduate Publications:
7. Harmatys, K. M., Musso, A. J., Clear, K. J., Smith, B. D. (2016) Small molecule additive enhances cell uptake of 5-aminolevulinic acid and conversion to protoporphyrin IX. Photochem. Photobiol. Sci. Advance Article, DOI: 10.1039/C6PP00151C
Link
6. Rice, D. R., Clear, K. J., Smith, B. D. (2016) Imaging and therapeutic applications of zinc(ii)-dipicolylamine molecular probes for anionic biomembranes. Chem. Commun. 52, 8787-8801 Link
5. Clear, K. J., Virga, K., Gray, L., and Smith, B. D. (2016) Using membrane composition to fine-tune the pKa of an optical liposome pH sensor. J. Mater. Chem. C 4, 2925-2930 Link
4. Clear, K. J., Harmatys, K. M., Rice, D. R., Wolter, W. R., Suckow, M. A., Wang, Y., Rusckowski, M., and Smith, B. D. (2016) Phenoxide-bridged zinc(II)-bis(dipicolylamine) probes for molecular imaging of cell death. Bioconjugate Chem. 27, 363-375 Link
3. Clear, K. J. and Smith, B. D. (2015) Synthetic Receptors for Polar Lipids. Synthetic Receptors for Biomolecules, Monographs in Supramolecular Chemistry, Royal Society of Chemistry: pp 405-436 Link
2. Plaunt, A. J., Clear, K. J., and Smith, B. D. (2014) 19F NMR indicator displacement assay using a synthetic receptor with appended paramagnetic relaxation agent. Chem. Commun. 50, 10499-10501 Link
1. Clear, K. J., Stroud, S., and Smith, B. D. (2013) Dual colorimetric and luminescent assay for dipicolinate, a biomarker of bacterial spores. Analyst 138, 7079-7082 Link
April 2018: Chemistry Night at Murray Elementary School (featuring Gena Wilson and other chem students)

March 2018: Dr. Clear with Hannah Adamson and Shelby Wakefield by their poster at the Spring 2018 ACS National Meeting
